Introduction to Lithium-Ion Technology
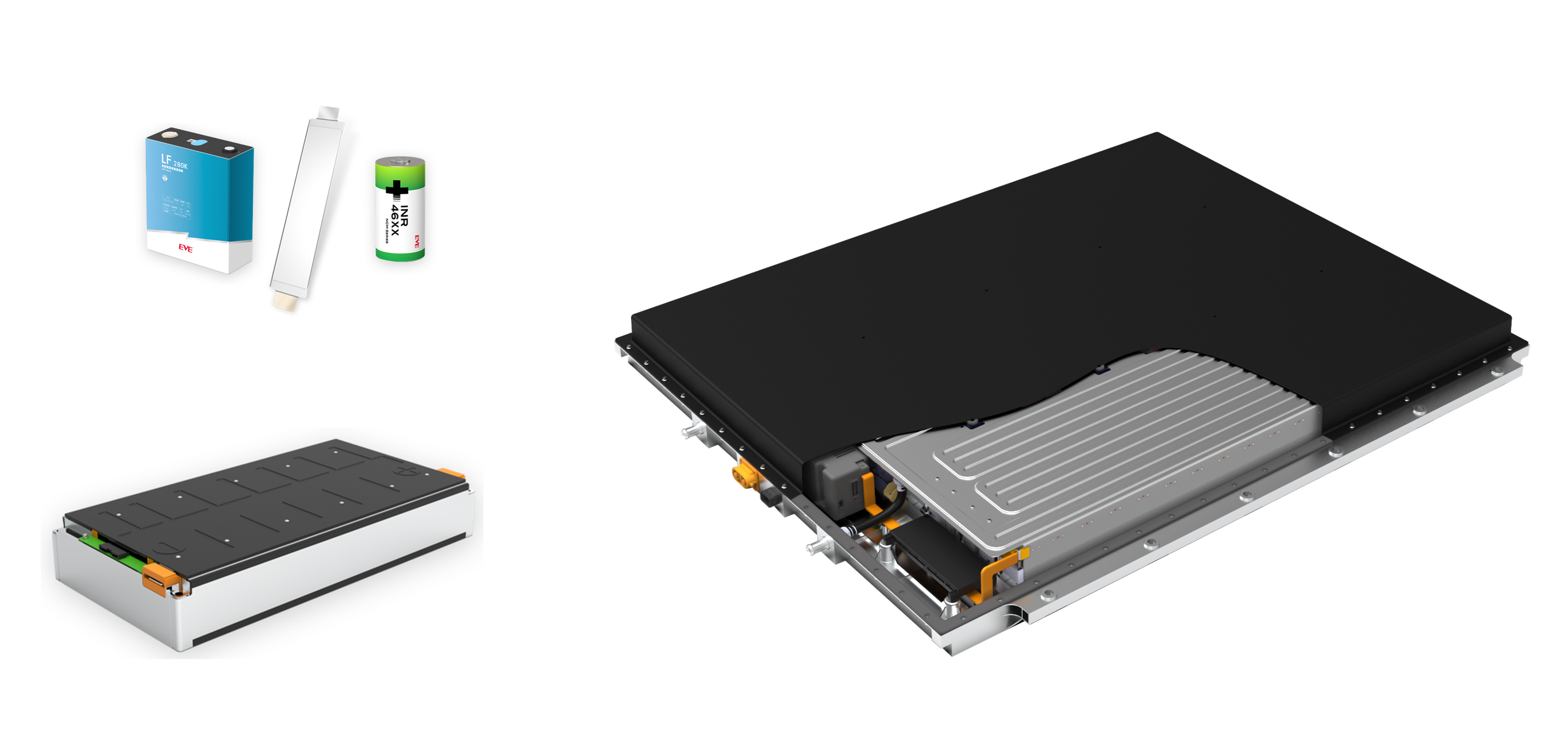
Fig. 1 – Cell, Module and Battery-Pack from EVE Energy
(Mit freundlicher Genehmigung/Courtesy of EVE Energy Co., Ltd. [Homepage])
Management Summary
The history of lithium-ion cells (Li-ion cells) began in the early 1970s – ironically enough – with the oil multinational Exxon, which was looking for new energy storage systems. In the years that followed, many scientists took up the results and developed them further. A highlight in the long history of research was the awarding of the Nobel Prize in Chemistry to the Americans Goodenough and Whittingham and the Japanese Yoshino in 2019.
The basic principle of Li-Io cells is based on the exchange of lithium ions between the anode and the cathode. During the discharging process, the lithium ions inside the cell move from the anode to the cathode, while the electrons outside the cell flow from the anode through the consumer to the cathode. This process is reversed during charging. As the lithium ions move back and forth, there is always an electrically neutral state at both poles.
A separator between the anode and the cathode prevents electrons from moving from one pole to the other within the cell to avoid a short circuit within the cell. However, the separator must be porous enough to allow the flow of lithium ions. An electrolyte is required so that the lithium ions can move within the separator. As lithium ions react very aggressively with oxygen, the electrolyte must only contain very small amounts of water.
Li-ion cells must have a variety of different properties so that they can be used in the automotive sector or in aviation. The most important performance parameters include energy density, cyclicity and, increasingly, fast-charging capability. Safety, industrial producibility, manufacturing costs and sales price also play an important role.
Many different cell chemistry variants for Li-Io cells have been investigated in the past, but two types currently dominate, which differ in terms of the cathode:
- Cells with a high energy density have a nickel-manganese-cobalt cathode; these cells are also called NMC cells (nickel, manganese, cobalt) in accordance with the English element names.
- In cells with a slightly lower energy density, the cathode consists of iron phosphate compounds; these are known as LFP cells. The disadvantage of the slightly lower energy density is compensated for by lower costs and greater robustness.
Lithium-ion cells also differ in terms of their form factor. Round cells and prismatic cells have a solid housing with a cylindrical or square geometry. The outer casing of pouch cells consists of a soft metal foil, which means that pouch cells are not dimensionally stable. Cells with all three designs are used in electric cars currently on the market.
The production of lithium-ion cells is complex and consists of three main steps.
- In electrode production, the anode and cathode are pre-produced in the form of foils, which requires time-consuming and energy-intensive drying phases.
- During cell assembly, the foils are cut into suitable individual pieces, which are then stacked (pouch cell, prismatic cell) or wound (round cell) and packaged in the housing. Finally, the liquid electrolyte is added.
- Cell finalization includes various measures to increase efficiency and quality assurance.
The lithium-ion batteries are assembled from the individual Li-ion cells . To do this, the cells are first integrated into modules . A battery then contains several modules, and the battery capacity can be configured quite easily based on the number of modules.
Research into innovative Li-ion cells is being carried out at universities, research institutes and start-ups around the world. In the short term, the focus is on the further development of the anode, and in the medium and long term on the change from a liquid to a solid electrolyte and the further development of the cathode.
History of lithium-ion cells
Research into lithium-ion cells (Li-ion cells) began at Exxon in the early 1970s. The background to this was the report published by the Club of Rome in 1972, which emphatically addressed the finite nature of the earth’s natural resources. The oil multinational was concerned that the petroleum industry’s business model could be jeopardized.
The British chemist M. Stanly Wittingham developed the first lithum ion cell at Exxon, in which the concept of reversible storage of ions in an atomic lattice was applied. Chemists refer to this as intercalation. At that time, the most energy-rich cells were made of nickel-cadmium compounds, which achieved a cell voltage of 1.3 volts, while the new battery cell from Wittingham already reached 2.4 volts.
At first, everything looked very promising. Exxon began producing button cells, which were used by a Swiss watch manufacturer, among others. But by the end of the 1970s, Exxon’s interest in this new technology had already faded. From then on, Exxon refocused on the oil business and sold the results of Wittingham as a license to three different battery manufacturers.
The US American Goodenough was familiar with Wittingham’s work. Goodenough had taken over as head of inorganic chemistry at Oxford at the end of the 1970s and continued to develop lithium-ion technology in England. Among other things, he changed the cell chemistry from titanium sulphide to lithium cobalt oxide, enabling the cell voltage to be raised to just under 4 volts.
The marketing of the further developed lithium-ion cells was less successful. Goodenough approached various battery manufacturers for cooperation, but they all turned him down. In the end, his work was patented by the British nuclear research institute AERE, with Goodenough having to cede all license income to AERE.
The baton was picked up again in Japan. There, Akiri Yoshino had investigated Li-Io cells with polyacetylene anodes at Asahi Chemical. On his last day at work in 1982, he came across a publication by Goodenough that had been lying unread on his desk. In the years that followed, he combined Goodenough’s cathode with various anodes, eventually ending up with the graphite anode.
The combination of lithium cobalt oxide and graphite proved to be much more stable than all previous variants. All that remained was to find a production process for lithium-ion cells that was suitable for series production. It turned out to be a stroke of luck that Sony was looking for new, more powerful batteries for its new camcorder.
At the beginning of 1987, Yoshino presented his new cell to the electronics group, the importance of which was immediately recognized by the Sony technicians. Under the leadership of Yoshio Nishi, the Sony team worked with suppliers to develop binders, electrolytes, separators and additives. They designed their own processes for the heat treatment of the anode and for the production of cathode powder in large quantities.
This paved the way for their triumphant advance. Lithium-ion batteries spread further via the Sony camcorder and found their way into laptops, cell phones and smartphones.
Until the day in the early 2000s when the two Tesla founders Martin Eberhard and Marc Tarpenning had the simple but ingenious idea of connecting a large number of lithium-ion cells together to build an electric car. But that’s another story.
Sources
Basic principle of lithium-ion cells
The basic principle of Li-Io cells is based on the exchange of lithium ions between the two cell poles during the charging and discharging process. This exchange ensures that the cell poles are always in an electrically neutral state. These are referred to as the anode and cathode, with the anode forming the positive pole and the cathode the negative pole.
- The anode contains graphite, in which the lithium ions are stored between the carbon compounds during the charging process. This reversible storage of ions in an atomic lattice is known as intercalation . However, this is not a chemical compound as we know it from chemistry lessons.
- The cathode consists of metal compounds, and two cell chemistry variants dominate in batteries for BEV vehicles. The first is based on nickel-manganese-cobalt compounds and is referred to as NMC in accordance with the element names. The second consists of lithium-iron-phosphate compounds and is abbreviated as LFP. You can find out more about the advantages and disadvantages of both types in the section on cell chemistry variants.
The substances used in the anode and cathode are also referred to as active materials because they are primarily responsible for energy storage. All other materials such as the outer shell, the separator and the electrolyte are summarized under the term passive materials.
Lithium ions consist of protons, i.e. the nucleus of the lithium atom. As the atomic nucleus of lithium contains only three protons, it is comparatively small and can therefore move back and forth between the poles within the cell. Lithium ions are positively charged and therefore enable charge equalization at the cell poles.
- During a discharge process, the electrons flow from the anode through the electrical load – such as an electric motor – to the cathode. The lithium ions stored in the graphite of the anode dissolve and migrate through the separator to the cathode. As the name suggests, the separator separates the two poles so that there is no flow of electrons within the cell. However, the separator must be porous enough to allow the flow of lithium ions.
- During a charging process, the electrons flow from the cathode via the charger into the anode. At the same time, the lithium ions move within the cell from the cathode through the separator to the anode.
In order for the lithium ions to be able to move within the separator, an electrolyte is required, which in principle can be solid or liquid. Liquid electrolytes based on solvents in which conductive salts containing lithium ions are dissolved are state of the art. The solvent may only contain very small amounts of water, as the lithium ions are very reactive with respect to the oxygen bound in the water.
During the first charging processes, a special layer called the solid electrolyte interface (SEI) forms in the transition area between the separator and the anode. It is formed from various substances dissolved in the electrolyte; the exact electrochemical processes are not yet fully understood and are the subject of research.
What is clear is that the SEI plays an important role in protecting the anode from the electrolyte; without this layer, the carbon-containing anode would quickly be destroyed. At the same time, the SEI also contributes to cell ageing because the SEI layer becomes thicker with increasing age, making the flow of Li-ions increasingly difficult.
Sources
https://e-lyte.de/de/wissen/funktionsweise-lithium-ionen-batterie/
https://www.scinexx.de/news/energie/raetsel-der-passivierungsschicht-im-akku-geloest/
Properties of lithium-ion cells
Li-ion cells must have a variety of different properties in order to be used in cars or airplanes.
The most important properties include performance parameters such as energy density, cyclicity and, increasingly, the fast-charging capability of Li-ion cells.
Other criteria include safety, industrial producibility and sales price.
The energy density of lithium cells can be measured both in relation to the cell weight and the cell volume. The first parameter is known as the graviometric energy density, the unit of measurement is Wh/kg. The second parameter is the volumetric energy density, which is measured in Wh/l.
The two quantities have different relevance depending on the area of application.
- Both values are important for passenger cars, as a low total weight is important and the installation space is very limited. This means that the cells should have a high graviometric and volumetric energy density.
- For larger vehicles such as trucks or buses , a slightly larger battery volume – i.e. a lower volumetric energy density – can be accepted because more installation space is available than for cars.
- For forklift trucks, a high battery weight – i.e. a low graviometric energy density – is expressly desired as a counterweight to the payload.
- For aircraft such as electric vertical take-offand landing vehicles (eVTOL), a low cell weight is an absolute priority.
Today’s standard cells with NMC cell chemistry used in the automotive sector achieve a gravimetric energy density of 220 – 240 Wh/kg and a volumetric energy density of approx. 500 Wh/l.
Cells with an even higher energy density are used in aviation. For example, the US start-up Amprius produces cells with 450 Wh/kg and 1150 Wh/l.
Although further increases are expected in the future, Li-Io cells will not reach the energy density of conventional fuels such as gasoline in the next few years. At 11,000 Wh/kg and 8500 Wh /l respectively, these are well above the values of the best Li-Io cells.
The cyclicity indicates how often cells can be charged and discharged. Compared to laptops or smartphones, cars have a very long service life, so the cyclicity requirements are higher.
Standard cells with NMC cell chemistry can be charged up to 1,500 times, which is absolutely sufficient for the typical service life of an electric vehicle. Cells with LFP cell chemistry can even be recharged up to 3,000 times.
The fast-charging capability of Li-Io cells is becoming increasingly important as the range of electric vehicles increases and DC fast-charging stations are expanded. While many BEV models such as the Smart EV, Renault Zeo or Nissan Leaf were primarily designed for short-distance driving at the beginning of electrification and were only equipped with slow AC chargers, most new electric cars today are equipped with DC fast chargers and are therefore suitable for long freeway journeys.
The fast-charging capability of Li-Io cells is therefore becoming a competitive factor. This is measured with C-rate, where 1 C stands for full charging in one hour. At 2 C, the cell can be fully charged in half an hour.
Today’s standard cells have a C-rate of 1-2 C. Cells that can be charged even faster are currently being developed by all cell manufacturers. The Israeli start-up StoreDot has made fast-charging capability its primary development goal.
Ultimately, it is not just the fast-charging capability of the cell alone that is decisive, but that of the entire battery system.
The central challenge with regard to the safety of Li-ion cells is the prevention of overheating and the associated potential self-ignition of the cell (thermal runway). The cell can set the neighboring cells and thus the entire battery on fire; a Li-ion battery that has caught fire cannot be extinguished using conventional means. The state of the art is to burn out or store the burning battery or vehicle in a water bath.
To prevent overheating, Li-ion cells in an electric car are always cooled with the help of a thermalmanagement system, with additional protection provided by the cell and battery housing.
Industrial producibility means that Li-Io cells can be produced in a highly automated process according to the assembly line principle with as little waste as possible. This is the only way to achieve mass production at an acceptable price.
The production of Li-Io cells is complicated, and the foundations for today’s established production processes were developed by Yoshio Nishi and his team at Sony .
The sales price of Li-ion cells has fallen sharply in recent years. According to a Bloomberg study, average prices in 2023 were $89 per kWh at cell level and $128 per kWh at battery level.
Battery prices are forecast to fall to $113 per kWh in 2025 and $80 per kWh in 2030.
Sources
https://www.flashbattery.tech/de/lithium-batterien-arten-welche-chemie-verwenden/
https://ecomento.de/2023/12/06/analyse-preise-fuer-lithium-ionen-akkupakete-erreichen-rekordtief-von-139-kwh
Cell chemistry variants for lithium-ion cells
In the more than thirty years of development of lithium-ion technology, a large number of cell chemistry variants have been developed and tested. Two variants have become established in the automotive industry; the cells are referred to as NMC or LFP cells according to the chemical elements (English names).
NMC cells have become the standard technology in the automotive sector. In NMC cells, the cathode consists of nickel-manganese-cobalt compounds, other elements of this cell chemistry are lithium and oxygen, the general chemical formula is LiNixMnyCozO2. The respective proportions of the three metals can vary, which is expressed by the parameters x, y and z in the formula.
In abbreviated form, the quantity ratio is indicated by a numerical code, here are a few examples:
- NMC111 means an equal distribution of 33 1/3 % each
- NMC811 stands for a proportion of 80% nickel, 10% manganese and 10% cobalt
As a large proportion of cobalt is mined in the Congo in Africa under ethically questionable conditions, cell manufacturers are endeavoring to reduce the proportion of cobalt in lithium-ion cells as much as possible. NCM811 has therefore become one of the leading cell chemistry variants.
SK On has reduced the cobalt content even further; the Korean manufacturer produces cells with a cobalt content of just 5%.
NMC cells basically offer a good compromise in terms of the key performance criteria. They have a high energy density (150-250 Wh/Kg) and a high cyclicity (1000-1500), and can be operated safely if a thermal management system is used. NMC cells are therefore the quasi-standard in the automotive industry.
LFP cells have established themselves in recent years as a low-cost alternative to NMC cells. The cathode of LFP cells consists of iron phosphate compounds, the chemical formula is LiFePO4. Rare and therefore expensive metals such as nickel, manganese or cobalt are not required.
LFP cells have a lower energy density (90-120 Wh/Kg), but a very high cyclicity (3000). The risk of self-ignition is lower than with NMC cells, as LFP cells have a higher ignition temperature.
Due to their lower costs, LFP cells are increasingly being used in the automotive industry for entry-level models, for example by Tesla and BYD.
Sources
Form factors for lithium-ion cells
There are three different form factors for lithium-ion cells, all three variants are used in electric cars:
- Round cells and prismatic cells have a solid housing with a cylindrical or square geometry.
- Pouch cells have a metal foil made of aluminum as a soft outer shell and are therefore not dimensionally stable.
Cells with a fixed housing offer fundamental advantages in terms of safety, as they protect the cell better against external interference. In the event of a sudden increase in pressure inside the cell, the neighboring cells are better protected. If the internal pressure exceeds the load limit of the cell housing, the internal pressure is reduced via a pressure relief valve.
The soft casing of pouch cells offers only very little protection; the cell can burst if the pressure rises. However, pouch cells have a lower weight.
Round cells were originally developed for portable electrical devices such as laptops, cell phones and smartphones. The length and diameter of round cells is standardized and is described with a sequence of numbers. For example, cells of type 18650 have a diameter of 18 mm and a length of 650 mm.

Fig. 2 – Round cells from Panasonic
(Mit freundlicher Genehmigung/Courtesy of www.akkushop.de [Homepage])
18650 cells such as those shown in Fig. 2 have been produced in very large quantities by various manufacturers for many years. In the early 2000s, Martin Eberhard and Marc Tarpenning had the simple but ingenious idea of connecting 6831 cells of this type together and installing them in the Tesla Roadster. You can read the full story here.
Other common round cell formats are 2170 (diameter 21 mm, length 70 mm) and 4680 (diameter 46 mm, length 80 mm), and there is a general trend towards larger cells in the automotive sector.
The standardization of round cells offers great advantages for vehicle manufacturers. Car manufacturers generally do not want to be dependent on one supplier. Therefore, if possible, they use the so-called second-source strategy, i.e. they purchase the same component from two different suppliers in order to ensure competition. This purchasing strategy is generally applicable to Li-Io round cells.
Prismatic cells are produced in a wide variety of sizes and shapes. Due to their mostly rectangular shape, prismatic cells can be interconnected more compactly than round cells.

Fig. 3 – Battery from BYD with blade cells
(Mit freundlicher Genehmigung/Courtesy of BYD Company Ltd. [Homepage])
Prismatic cells can be much larger than round cells. An extreme example is BYD‘s blade cell, which reaches a length of almost one meter (Fig. 3). Larger cells naturally also mean fewer components in the battery and therefore less complexity.
In contrast to round cells, there is no form standard on the market for prismatic cells. Large manufacturers such as VW, who plan their own cell production at the daughter company PowerCo, define their own “in-house standard” themselves. The “unified VW cell” has a length of approx. 34 cm, a height of approx. 14 cm and a thickness of approx. 3 cm, whereby the exact dimensions are not communicated by VW.

Fig 4 – Various cells in pouch format from LG Energy Solution
(Mit freundlicher Genehmigung/Courtesy of LG Energy Solution Europe GmbH [Homepage])
Pouch cells have advantages above all in terms of weight and production costs. The lightweight aluminum foil shell provides maximum flexibility in terms of cell size (Fig. 4), which is why the pouch format is often used for cell prototypes in early sample phases.
The main disadvantage of pouch cells is their low safety, as the lightweight aluminum foil offers neither good protection “from the outside” nor “to the outside”. Sharp objects can easily penetrate the foil and cause a short circuit. Li-Io cells expand during charging and the aluminum foil can easily burst.
Pouch cells must therefore always be installed in modules with a stable housing, which reduces the weight advantage of pouch cells.
Of course, the question arises as to whether all three designs will continue to be used alongside each other in the future or whether one variant will dominate.
In the automotive sector, there is a trend towards cells with a fixed housing. However, it is impossible to predict which of the two variants, i.e. round cell or prismatic cell, will dominate in the future.
- Tesla, formerly the largest BEV manufacturer, started out with round cells, but also uses prismatic cells from BYD. BYD itself sells round cells as a battery manufacturer, but primarily installs prismatic cells in its vehicles.
- Volkswagen relies on the prismatic format for its standard cell, while BMW is planning to switch from prismatic cells to round cells (“new class”). As BMW does not produce its own cells, the second-source advantage of round cells may have been the decisive factor.
- Ford, GM and Mercedes-Benz have so far relied on pouch cells, although GM has announced a switch to round cells.
Ultimately, the question of the future dominant design is less decisive for competition than the question of future cell chemistry.
Sources
Production of lithium-ion cells
The production of lithium-ion cells is complex and consists of the three main steps: electrode production, cell assembly and cell finalization.
Electrode production is used for production preparation and must take place in clean rooms, as the smallest dust particles can affect the cell quality.
In the first step, the active materials for the anode and cathode are mixed together separately. In both cases, solvents are added to obtain a viscous substance known as slurry .
The slurry is then applied to the respective carrier film for the anode and cathode. The film is supplied in roll form; the slurry is applied by unwinding the film and feeding it past a dosing machine. The carrier foil of the anode is made of copper, that of the cathode of aluminum.
The applied active material is then compacted by rolling and dried. Finally, the base foil (mother coil) is cut into foils with a smaller width and rolled up (daughter coils).
The film rolls are then stored in drying cabinets, where the last remaining moisture is removed. This process can take up to 48 hours.
The actual assembly of the cells takes place during cell assembly. This must take place in dry rooms, as water in the cell leads to a severe loss of quality.
Cell assembly differs depending on the design.
- In the case of pouch cells and prismatic cells , the daughter coils are unrolled and cut into suitable individual pieces, which are then stacked together with the separator foil. Many stacking technologies are manufacturer-specific and patented.
- In round cells , the anode foil, the separator foil and the cathode foil are placed on top of each other and then rolled up.
Finally, the cell stacks or the rolled-up cell are packed into the housing, fitted with electrical connections and the liquid electrolyte is poured in. In order to ensure that the electrolyte spreads well in the cell, a vacuum is created in the cell.
Cell finalization is the last production step. This can take place in normal industrial rooms, as the cell is already sealed.
In the first step, the cells are placed in a rack system and heated to 30 – 50 degrees. This makes the electrolyte more fluid and allows it to spread even better in the separator.
The cells are then charged and discharged according to precisely defined current and voltage curves, a process also known as forming . In this step, lithium ions are stored in the graphite of the anode for the first time. The so-called solid electrolyte interface (SEI) forms in the contact area of the anode and separator, protecting the graphite from the electrolyte in the separator.
During the degassing phase, the cell is opened again to remove the gases. Depending on the form factor, i.e. cylindrical, prismatic and pouch, the degassing processes differ.
One of the last steps is the so-called maturation. Here the cells are charged and discharged under different temperature conditions for up to 3 weeks and monitored. In particular, the cells are also run through the permissible low and high temperature ranges.
All cells that survive the maturing process undergo a final end-of-line test (EoL test), in which various measurements and tests are carried out.
Cells that have passed the EoL test are then packaged and delivered to the customer.
Sources
PRODUCTION PROCESS OF A LITHIUM-ION BATTERY CELL, RWTH Aachen University: www.pem.rwth-aachen.de, VDMA: vdma.org/batteryproductionmeans
Lithium-ion batteries
Lithium-ion batteries consist of modules in which the lithium-ion cells are interconnected (Fig. 1).
Vehicle manufacturers can configure the battery capacity quite easily via the number of modules installed in the battery. For entry-level variants of a model series, spaces in the battery simply remain unoccupied, which reduces the battery price.
This modular battery concept is used by many manufacturers, for example by Mercedes-Benz in the EQA and EQB models.
In the modules, each cell is monitored individually in order to regulate the fill level of the cells. The aim is to load all cells as equally as possible in order to avoid premature ageing of individual cells.
The term state-of-charge (SoC) has become established for fill level, while the state of ageing is referred to as state-of-health (SoH).
A thermal management system regulates the operating temperature of the cells, which ideally lies within a thermal window of 20 – 40 degrees.
The entire battery is monitored by a so-called battery management system (BMS). The BMS is an electronic control unit similar to the engine control unit of a combustion engine. The BMS communicates with the electric vehicle via a bus system and controls all processes relating to the battery.
In recent years, larger cells have reinforced the trend towards installing cells directly in the battery and dispensing with the intermediate module level.
A well-known example is BYD‘s blade battery (Fig. 3), which is equipped with cells that are almost 1 m long. Eliminating the module level improves the energy density of the battery, as less passive material is required for the module housing and module electronics.
Research into innovative lithium-ion cells
Research into Li-ion cells is being conducted worldwide at both the upper and lower end of the performance spectrum.
- Work at the upper end addresses the further increase in energy density and fast-charging capability, with a focus on the further development of the anode and the use of a solid instead of liquid electrolyte.
- Work at the lower end is focused on reducing costs, with the most radical approach being based on replacing the raw material lithium with sodium.
Sodium cells have a significantly lower energy density than NMC cells, but are much cheaper due to the virtually limitless availability of sodium as a raw material.
Sodium cells are being developed by CATL and others. The world’s largest cell manufacturer forecasts a price of 3,000 – 4,000 euros for a 50 kWh battery, which represents a cost advantage of 50% over a lithium battery. The German professor Markus Lienkamp assumes that LFP batteries will be completely replaced by sodium batteries by 2030.
At the upper end of the performance spectrum, the main focus is on increasing the energy density and improving the fast-charging capability of Li-ion cells.
In the short term, measures are aimed at further developing the anode, while in the medium to long term the focus is on switching from a liquid to a solid electrolyte and further developing the cathode.
In the short-term further development of the anode, the aim is to increase the amount of lithium ions that can be stored in the graphite-based anode.
It has been known for some time that compounds with the element silicon can store up to ten times more lithium ions than graphite. The problem is the very strong mechanical expansion of silicon when Li-ions are incorporated. Too much silicon can cause the anode to swell considerably and thus destroy the cell.
Various technologies are being investigated to get to grips with this problem. One approach is silicon enrichment in the form of tiny particles.
- The US start-up Amprius works with extremely thin silicon wires with diameters in the nano-meter range. According to its own information, Amprius is the first cell manufacturer to commercialize lithium-ion cells with a silicon anode; the target market is aviation
- The US start-up OneD also relies on silicon nanowires that are mixed with graphite. In June 2023, a technology partnership was announced with the German small series manufacturer Customcells.
- The Israeli start-up StoreDot has developed nano-sized silicon grains, from which the company name is derived (dot stands for point or particle). StoreDot’s primary goal is to improve fast charging capability. Partners include Samsung and Mercedes-Benz, and the cells are to be produced by EVE Energy.
Another approach is the development of materials that encapsulate the silicon in order to mechanically limit its expansion. The US start-up Blue Current, for example, is pursuing this approach.
Cells with slightly silicon-enriched anodes are close to market maturity, for example in the electric Mercedes-Benz G-Class announced for 2024. The cells come from CATL, while the supplier of the anode material is the US start-up Sila.
As an alternative to silicon, the use of lithium metal compounds as anode materials is also being investigated. Here, too, there are a number of risks to consider. In addition to the problem of mechanical expansion, dendrite formation can occur. These needle-like structures on the anode can penetrate the separator and cause a short circuit.
Examples of start-ups working on this technology with the support of automotive companies include
- QuantumScape, in which VW is involved and
- Factiorial Energy with Mercedes-Benz, Stellantis and Hyundai as technology partners.
In the medium term, research will focus on replacing the liquid electrolyte with a solid electrolyte.
The three best-known approaches include polymer-, oxide– and sulphide–based solid electrolytes. Each of these three technologies has its advantages and disadvantages, and none of the materials investigated to date has been able to fulfill all the properties of today’s standard cells. The cells often achieve a high energy density but have a low cyclicity. If both parameters are right, there is still no industrial production process.
- Blue Solutions – The French company relies on lithium-polymer solid electrolytes in combination with a lithium-metal anode. Blue Solutions describes itself as a pioneer of solid-state cells, as these are already used in Mercedes-Benz city buses. However, the cells must be operated at 50-80 degrees, as the conductivity of the electrolyte is only given in this range.
- ProLogium – The Taiwanese start-up has been working on oxide-based solid-state cells since 2006, and a European production facility is currently under construction. Its technology partners include Mercedes-Benz.
- Solid Power – The US start-up is developing cells with a sulphide-based solid electrolyte in combination with a silicon anode; technology partners include BMW.
Interestingly, there are also start-ups such as SES AI that have stopped working on solid-state cells due to the many difficulties and have returned to liquid electrolytes.
The interview with Markus Schäfer published in the British newspaper Autocar in January 2024 also caused a stir in this context. In it, the Chief Technology Officer of Mercedes-Benz explains that the progress made with conventional lithium-ion batteries is so high that they are “neck and neck” with solid-state batteries in terms of cost and energy density. Perhaps solid-state cells are therefore not necessary at all.
Comparatively few start-ups are working on cathode innovations that go beyond variations of the chemical variants NMC and LFP:
- The US start-up Volexion produces drop-in material for the cathode based on a graphene coating. The material is designed to increase the cyclicity of the cell by 100%.
- The US start-up Lyten also uses graphene for the development of cathodes; specifically, it is used together with a sulphur-lithium compound.
- The German start-up Theion is developing cells with a sodium-sulphur cathode and is talking about a crystal battery.
Summary: We currently expect evolutionary rather than revolutionary developments in the field of lithium-ion technology in the short term.
However, in terms of progress in electromobility, we are happy to be surprised.
We would not be surprised if the surprise came from China.
